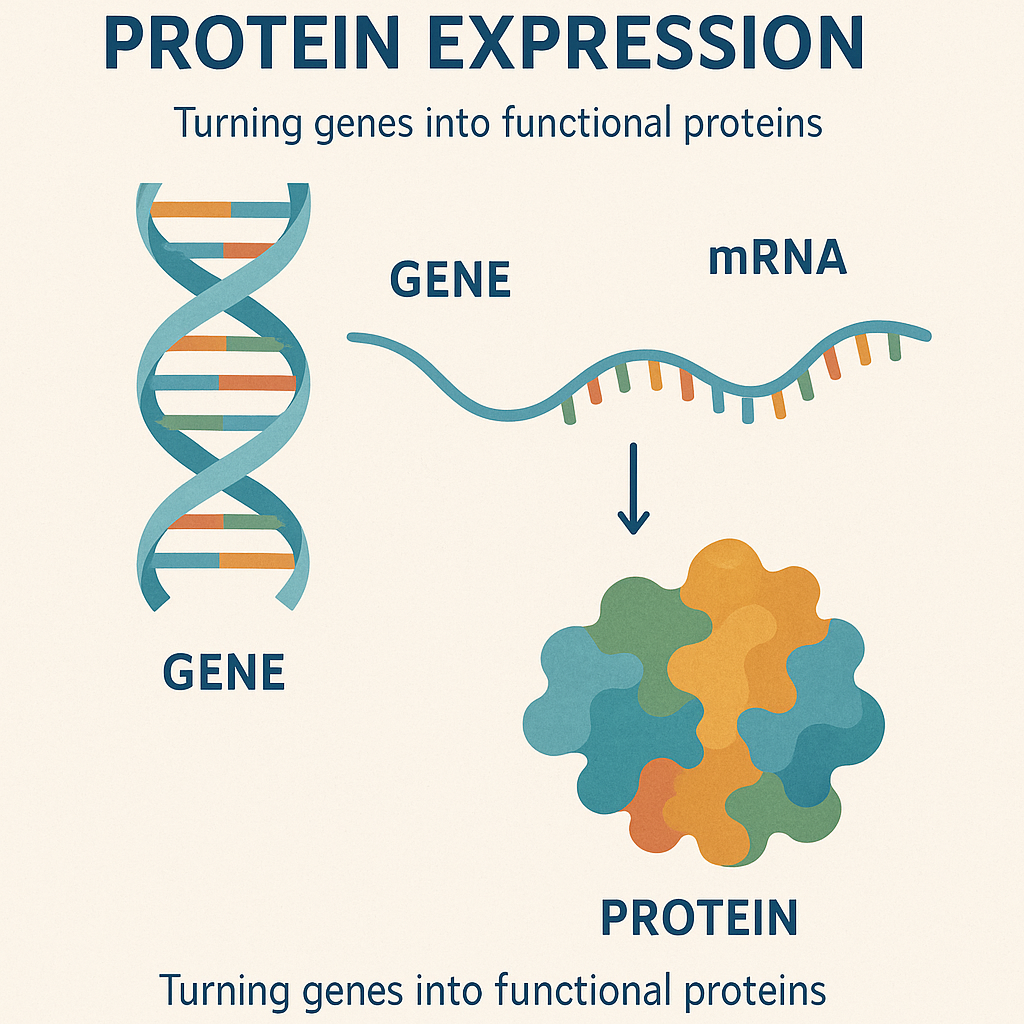
Protein expression is a fundamental process in biotechnology, enabling the synthesis of functional proteins from genetic templates for diverse applications, including therapeutic development, basic research, and industrial manufacturing. This complex biological process involves the translation of DNA-encoded sequences into proteins via a series of tightly regulated steps.
These steps are often complemented by advanced purification technologies such as fast protein liquid chromatography (FPLC), which ensure molecular integrity and functional activity. Herein, we provide a detailed overview of the molecular mechanisms underlying protein expression and discuss the technological advancements that facilitate efficient protein production.
Molecular basis of protein expression
Protein expression encompasses two central biochemical stages: transcription, during which DNA sequences are transcribed into messenger RNA (mRNA), followed by translation, wherein ribosomes decode the mRNA to assemble amino acid chains that fold into functional proteins. The pathway from gene to active protein is frequently complicated by challenges such as suboptimal expression levels, improper protein folding, and the necessity for specific post-translational modifications (PTMs), each requiring bespoke methodological interventions.
The key steps in protein expression are as follows:
- Gene cloning: Target gene sequences are isolated and cloned into suitable expression vectors—plasmid or viral DNA constructs—that facilitate gene delivery into host cells. Regulatory elements such as promoters (eg, T7 in Escherichia coli and CMV in mammalian systems) drive controlled transcription.
- Host selection: The choice of host organism is crucial and guided by protein complexity as well as desired PTMs. E. coli remains the workhorse for high-yield production of simple proteins, whereas mammalian cell lines such as HEK293 and Chinese hamster ovary (CHO) cells are preferred for producing proteins requiring glycosylation, disulfide bonding, and other complex modifications.
- Culturing and induction: Host cells are cultured under optimized physicochemical conditions—such as temperature, pH, and nutrient concentration—to maximize expression yields. Inducers such as isopropyl β-D-1-thiogalactopyranoside (IPTG) in bacterial systems and tetracycline derivatives in mammalian systems initiate transcription at defined growth phases.
- Harvesting and purification: After expression, cells undergo lysis to release intracellular proteins. Downstream purification commonly makes use of chromatographic techniques, with FPLC representing the gold standard for separating proteins based on physicochemical properties such as size, charge, and affinity.
Choosing the right expression system
The choice of expression system depends on the protein’s complexity, required yield, and intended use. Each system has its distinct advantages and limitations.
Prokaryotic systems
E. coli and related bacterial hosts offer rapid growth kinetics, ease of genetic manipulation, and cost-effective media requirements, making them ideal for producing non-glycosylated proteins, including enzymes and antibody fragments. Nonetheless, their inability to perform eukaryotic PTMs limits their suitability for many therapeutically relevant proteins.
Eukaryotic systems
Mammalian expression systems, especially HEK293 and CHO cells, provide authentic PTMs, such as glycosylation patterns, essential for biological activity and immunogenicity of therapeutic proteins, such as monoclonal antibodies. While these systems yield high-quality proteins, their slower growth rates and higher operational costs must be considered.
Yeast and insect cell systems
Yeast species such as Pichia pastoris and insect cell lines utilizing baculovirus vectors offer intermediate solutions combining the scalability of prokaryotes with partial eukaryotic PTM capabilities. These hosts are valuable for producing glycosylated proteins and viral glycoproteins, particularly in vaccine development.
Addressing key challenges in protein expression
Protein expression is fraught with challenges, but innovative strategies have emerged to address them.
Low protein yield
Variability in codon usage between heterologous genes and host translation machinery often results in inefficient protein production. Codon optimization algorithms, which redesign genes for host-preferred codons, significantly enhance expression efficiency. In addition, optimizing induction parameters such as temperature reduction can improve protein folding and stability, mitigating aggregation.
Protein misfolding and aggregation
Recombinant proteins, particularly those with complex tertiary structures such as membrane receptors and antibodies, frequently misfold, forming insoluble aggregates known as inclusion bodies. Strategies to overcome this include the co-expression of molecular chaperones (eg, GroEL-GroES systems in E. coli), and in vitro refolding protocols utilizing denaturant gradients to recover functional conformations.
Post-translational modifications
Proteins requiring PTMs, such as erythropoietin (glycosylation) or insulin (disulfide bond formation), necessitate eukaryotic hosts to achieve biological activity. Alternatively, emerging cell-free protein synthesis platforms enable precise control over PTMs and the incorporation of non-canonical amino acids but remain limited by scale and cost.
Removal of contaminants
Ensuring product purity is paramount for biopharmaceutical applications. Contaminants such as host cell proteins, DNA, and endotoxins are effectively separated using FPLC, which can exploit affinity tags (eg, polyhistidine tags) and ion-exchange chromatography for high-resolution purification.
Fast protein liquid chromatography: Purification excellence
FPLC remains important for protein purification owing to its ability to maintain protein integrity under gentle conditions and simultaneously provide high resolution and reproducibility. This method employs biocompatible buffers and chromatographic resins to separate proteins on the basis of multiple parameters, including size exclusion, ionic charge, and affinity interactions.
Operational principles
- Binding: The crude protein mixture is loaded onto a column packed with resin beads functionalized for specific interactions, such as nickel ions capturing His-tagged proteins.
- Elution: Target proteins are selectively released by gradient elution using buffer compositions; eg, imidazole gradients for His-tag affinity purification.
- Detection: Protein elution is monitored via ultraviolet absorbance at 280 nm, and buffer conductivity measurements confirm gradient progression, ensuring precise fraction collection.
Advantages
- High purity: Capable of removing over 99% of contaminants, which is vital for therapeutic applications requiring stringent regulatory compliance.
- Scalability: Suitable for processing from milligram-scale research batches to multi-gram industrial productions.
- Versatility: Supports various chromatography modalities, enabling customized workflows for complex protein mixtures.
For instance, therapeutic insulin production employs FPLC-based size-exclusion and ion-exchange chromatography to achieve pharmaceutical-grade purity following bacterial expression.
Applications
Expressed proteins underpin a broad spectrum of biotechnological innovations:
- Therapeutics: Monoclonal antibodies (eg, pembrolizumab) have revolutionized oncology, while recombinant vaccines, including mRNA-based COVID-19 vaccines, demonstrate the impact of protein engineering in infectious-disease control.
- Research tools: The CRISPR-Cas9 system enables precise genome editing, and fluorescent reporters such as green fluorescent protein (GFP) facilitate real-time cellular imaging.
- Industrial enzymes: Enzymes such as cellulases enhance biofuel production by degrading plant biomass, and proteases are integral to detergent formulations to improve cleaning efficacy.
Emerging trends and future perspectives
Innovations are continuously advancing the field of protein expression:
- Cell-free expression systems: These platforms circumvent cellular limitations, allowing the synthesis of toxic and difficult-to-express proteins and enabling site-specific incorporation of unnatural amino acids to enhance protein functionality.
- Artificial intelligence (AI)-driven optimization: Machine learning algorithms now facilitate the design of optimized codon usage, folding pathways, and purification protocols, dramatically accelerating development timelines and reducing costs.
- Continuous manufacturing: Integration of protein expression and FPLC purification in automated, continuous-flow systems streamlines production workflows, enhancing efficiency and product consistency, along with minimizing contamination risks.
Conclusion
Protein expression serves as the critical nexus for translating genetic information into biologically active molecules, driving advancements from life-saving therapeutics to sustainable industrial applications. Despite inherent challenges, including yield optimization and proper folding, the continuous evolution of expression platforms and purification methodologies, such as FPLC, sustains robust progress in the field. As scientific innovation progresses, refining these technologies promises to unlock further potential in protein engineering and biopharmaceutical development.